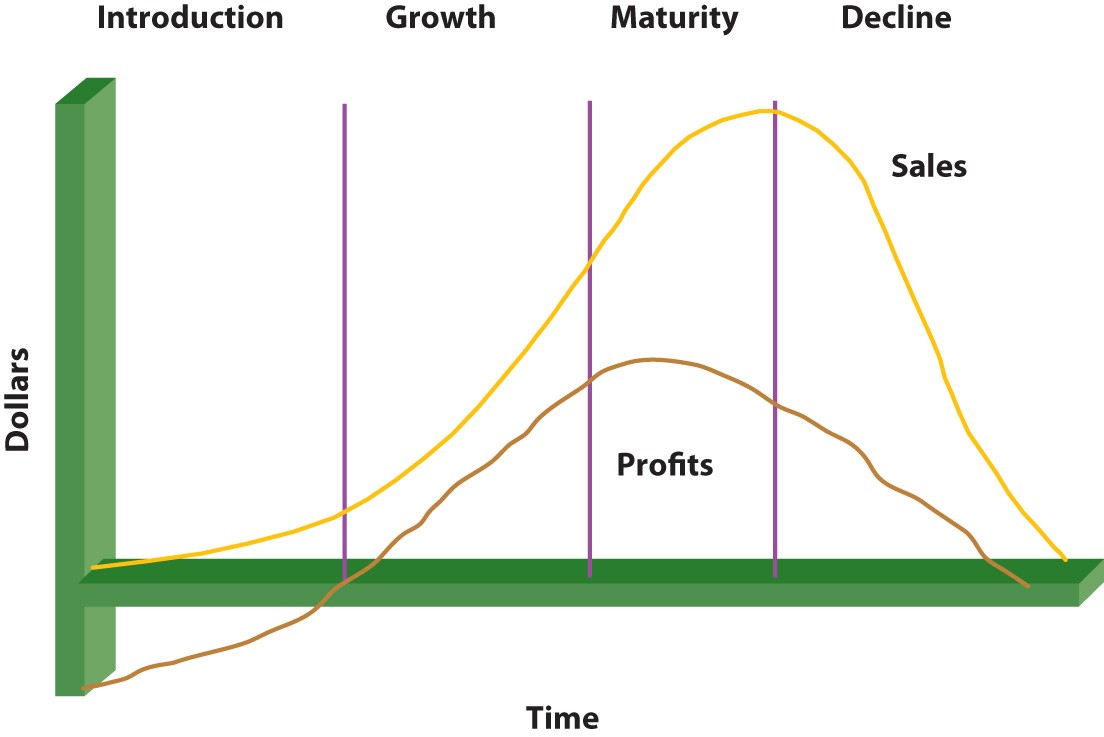
All products and services have certain life cycles. The term “life cycle” describes the time span between a product’s first release onto the market and its eventual withdrawal. Significant adjustments are made to the product’s market behavior during this time, which is reflected in the sales to the firm that brought it to the market. Understanding a product’s life cycle can assist a company in determining its position in the market relative to rivals and the success or failure of the product. But certainly, there are some challenges in the process.
The following are the main obstacles to pharmaceutical product lifecycle management that I have attempted to highlight in this article:
- Internal and external complexity are rising
The fact is that many pharmaceutical organizations experience information silos across many functional areas, which causes various challenges when managing the whole product lifecycle from product inception to phase out.
- There are multiple sources of information about products
Depending on the functional area, divergent, redundant, and erroneous product information is frequently a result of a range of diverse data sources and a lack of collaboration within the business.
- Research and Development improvement
Opportunities exist for innovative pharmaceutical companies looking to restructure their businesses and achieve profitability and growth in an environment that is becoming more and more competitive. Companies will experience enhanced company performance and differentiation in the market and technology if they properly manage the transformation process to meet these concerns.
- Technology transfer
Since the manufacturing process for pharmaceutical products is extremely iterative, control must be developed for each lot from scale-up to validation and quality assurance, and finally to the marketing of the final, approved product. Drug production must be scaled up effectively, which necessitates cooperation across a variety of interconnected activities and dependencies.
- Integrated quality and risk management
When flaws are discovered during regulatory audits, quality and risk management continue to be difficult tasks with substantial commercial ramifications. From product development to commercialization, quality must be integrated and managed as part of an efficient enterprise quality management system.
- Comprehensive packaging
Pharmaceutical packaging is strictly regulated and has to pay attention to ingredients, product quality, and adverse event labeling. Pharmaceutical firms must deal with various regulatory, linguistic, and counterfeit control needs as a result of global distribution. The commercial success of medicine depends heavily on cost management and keeping maximum flexibility in today’s fiercely competitive climate. The regulatory integrity of all related commercial content will be improved by creating a global archive for all package elements, digital assets such as logos and artwork, and marketing materials that reference development evidence.
- Registering products globally
This complicates the management of drug compound registration and is continually changing. This causes market launch delays and adversely affects the projected sales of the product.
- Portfolio of intellectual property
Clinical study and regulatory approval phases generally take up a major portion of a new drug’s patent protection period. Pharmaceutical companies must provide a portfolio of new pharmaceuticals and drug extensions to the market more swiftly in order to maintain their competitiveness and benefit from the patent protection period.
- Complex collaborative outsourcing networks management
To increase productivity, concentrate on their core competencies, and take advantage of the pooled expertise, pharmaceutical businesses increasingly rely on contract service providers. Collaborative talents enable the quick identification of problems with products and processes as well as the implementation of necessary modifications. By exchanging data with their product development department, businesses can remotely examine and approve outsourced components of products and projects.
- Corrective and Preventive Action (CAPA) System
Many pharmaceutical firms find it necessary to put additional quality control systems in place since they were responsible for producing a high-quality products. Companies need an infrastructure to identify current or potential quality issues in order to comply with CAPA regulations. By removing the need for manual documentation, enhancing the efficiency of root cause analysis, and streamlining time-consuming feedback procedures, having CAPA procedures online and tightly integrated with core product information cuts costs.